MYOFASCIAL TRIGGER POINTS
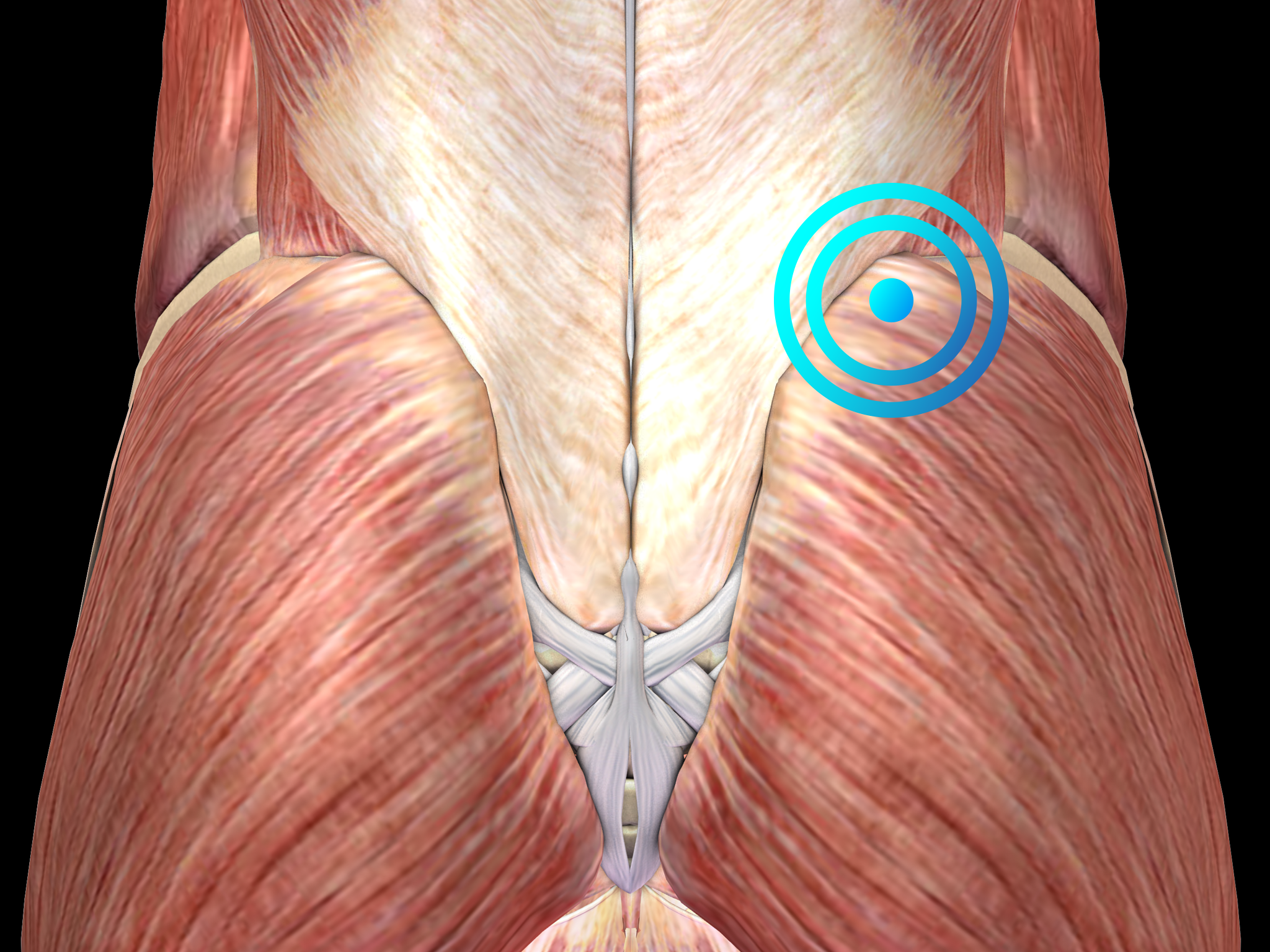
Pathology
Diagnosis is clinical and includes the identification of small knots (2-5 mm in diameter) and tight bands within affected muscles that are painful when palpated. Diagnostic imaging is not helpful and should only be considered to rule out other causes of muscle pain.
It is still unclear whether or not myofascial trigger points are true pathologic entities. It has been hypothesized that muscle injury or stress disrupts the sarcoplasmic reticulum within muscle fibers, releasing free calcium ions. These free calcium ions causes the actin and myosin of the muscle fibers to lock into place as long as adenosine triphosphate is available. The resulting contraction of small parts of the muscle leads to diminished blood flow with subsequent ischemia and release of painful substances such as serotonin, histamine and prostaglandins in the affected area.
Myofascial trigger points are a very common condition, especially in the cervical musculature. Up to 85% of back pain and approximately 55% of neck pain and headaches are caused by myofascial pain.
The predominant age of patients suffering from myofascial trigger pointsis 30 to 50 years. Women are more affected than men.
Very often Myofascial trigger points are associated with poor posture. Notably, myofascial trigger pointss frequently produce neurological complaints including headache, dizziness, sensory symptoms, as well as gastrointestinal problems.
The treatment of Myofascial trigger points should start with a manual technique that involves applying pressure to a trigger point to release the pathologic contraction of the muscle segment and to stretch the segment to restore normal muscle fiber length. This can be accompanied by acupuncture, stress management and relaxation techniques. Pharmacologic treatment is unspecific and may comprise muscle relaxants, nonsteroidal anti-inflammatory drugs (NSAIDs), anticonvulsants, or topical application of local anesthetics or botulinum toxin.
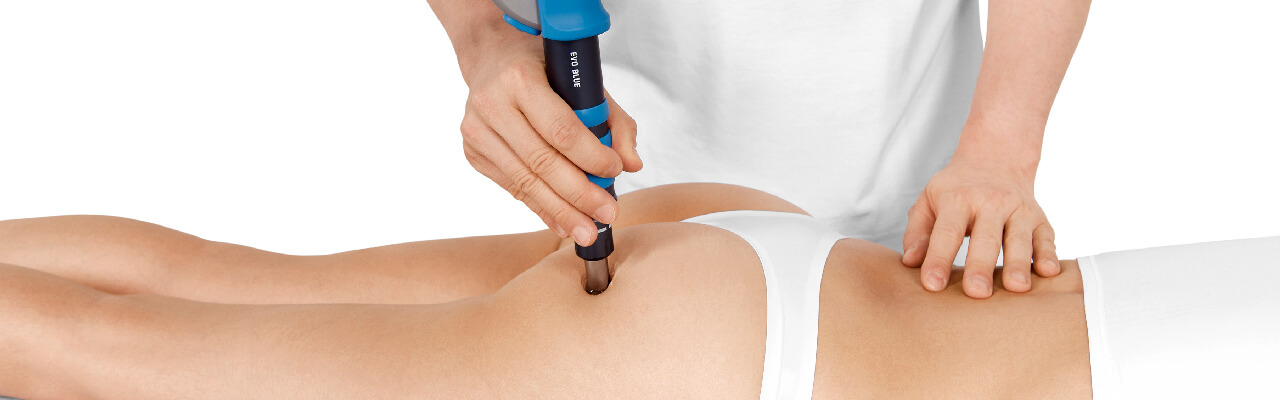
Radial shock wave therapy is very effective for Myofascial trigger points and may mimic manual therapy in applying pressure to a trigger point to release the pathologic contraction of the muscle segment.
Prevention of recurrence should focus on appropriate ergonomic changes in patients’ day-to-day activities to avoid repetitive stress to the injured muscles.
Side effects of Radial Shock Wave Therapy (RSWT) using the Swiss DolorClast®.
When performed properly, RSWT with the Swiss DolorClast® has only minimal risks. Typical device-related non-serious adverse events are:
- Pain and discomfort during and after treatment (anesthesia is not necessary)
- Reddening of the skin
- Petechia
- Swelling and numbness of the skin over the treatment area
These device-related non-serious adverse events usually disappear within 36h after the treatment.
Treatment Procedure
Clinical Proof
Stosswellentherapie beim idiopathischen Rückenschmerz pseudoradikulären Syndromen
Bauermeister W.
Maier m, Gillesberger F: Abstract 2003 zur Muskuloskelettalen Stosswellentherapie: Norderstedt, 2003, 29-34
This article shows how shock wave therapy can be used to treat idiopathic back pain pseudoradicular syndromes.
(German Study)
Recommended Settings
| Recommended Settings | Treatment |
| Number of treatment sessions | 3 to 5 |
| Interval between two sessions | 1 week |
| Air pressure Evo Blue® | 2.5 to 4 bar |
| Air pressure Power+ | 2 to 4 bar |
| Impulses | 500 - 1000 impulses per trigger point |
| Frequency | 12Hz |
| Applicator | 15mm or 15 mm trigger |
| Skin pressure | Light to Moderate |
Contraindications
The following contraindications of RSWT using the Swiss DolorClast® must be considered:
- Treatment over air-filled tissue (lung, gut)
- Treatment of pre-ruptured tendons
- Treatment of pregnant women
- Treatment of patients under the age of 18 years (except for Osgood-Schlatter disease and muscular dysfunction in children with spastic movement disorders)
- Treatment of patients with blood-clotting disorders (including local thrombosis)
- Treatment of patients treated with oral anticoagulants
- Treatment of tissue with local tumors or local bacterial and/or viral infections
- Treatment of patients treated with cortisone
Some indications may not be approved in the United States of America, under regulation by the US FDA. Please refer to the respective Instructions for Use.
