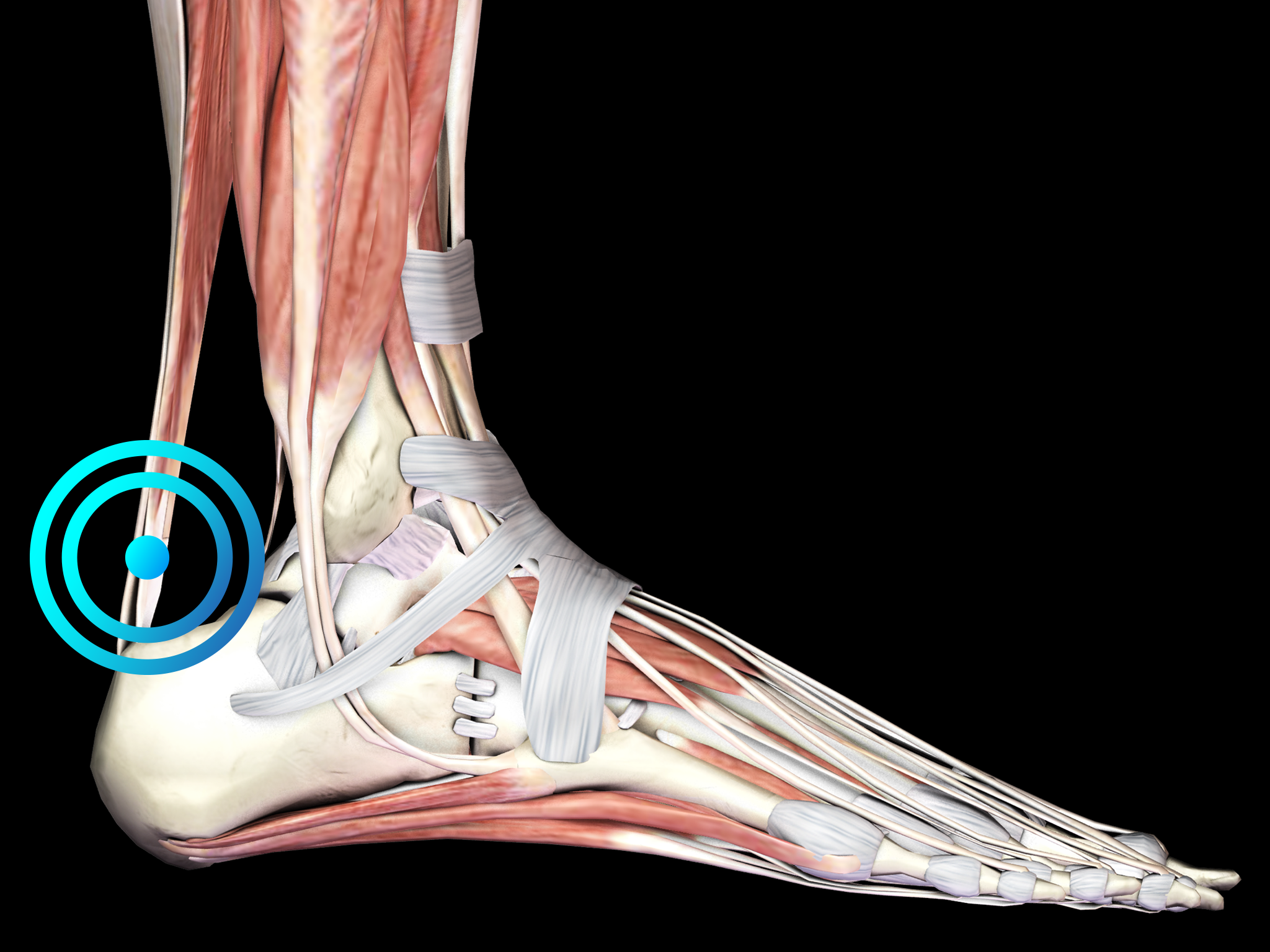
INSERTIONAL ACHILLES TENDINOPATHY
Pathology
However, histologic examination of biopsy specimens from patients undergoing surgery for chronic symptoms has shown that chronic insertional Achilles tendinopathy is associated with degenerative changes in the tendon. Accordingly, the disease is better characterized as tendinopathy than tendinitis or tendinosis.
Diagnosis is based on the clinical features of the disease, with the location of the pain as an important discriminating factor. The spot of maximum pain in IAT is located at the tendon-bone junction, whereas in the case of noninsertional Achilles tendinopathy, the spot of maximum pain is 2 to 6 cm proximal to the insertion. Symptoms can be exacerbated by running on hard surfaces and climbing stairs. Diagnostic imaging should be considered to rule out other causes of Achilles tendon pain and heel pain or to establish the diagnosis of IAT when in doubt.
The aetiology of IAT is likely multi-factorial and may include advanced age, obesity, hypertension, diabetes, hyperpronation and steroid use, to mention only a few.
Runners comprise the largest group of patients with chronic pain in the Achilles tendon. The annual incidence of insertional achilles tendinopathy among athletes is approximately 8%. However, individuals of all activity levels and all ages present with similar complaints.
Particularly in athletes, the onset of IAT may also be influenced by poor training habits including excessive training, training on hard or sloping surfaces, and abrupt changes in scheduling.
It has been hypothesized that healing of injuries of the Achilles tendon as a result of overuse involves the penetration of small blood vessels into the tendon in order to increase healing by providing improved blood flow. However, these small blood vessels are accompanied by small nerve fibers with high concentrations of nociceptive substances including glutamate, substance P, and calcitonin gene-related peptide (CGRP). Those small nerve fibers are considered the cause of pain in chronic insertional Achilles tendinopathy.
The treatment of insertional Achilles tendinopathy should start with conservative treatment modalities including rest, icing, physiotherapy, stretching (eccentric loading), exercises, orthoses, heel lifts and non-steroidal anti-inflammatory drugs. In certain cases, braces and immobilization with a cast or a pneumatic walking boot may improve the situation.
Patients not responding to conservative treatment for six months shall then undergo radial shock wave therapy for insertional achilles tendinopathy treatment.
Surgery should be considered for recalcitrant cases of insertional Achilles tendinopathy, with different surgical strategies described in the literature. Prevention of recurrence should focus on appropriate exercise habits, wearing low-heeled shoes and eccentric strengthening exercises.
Side effects of Radial Shock Wave Therapy (RSWT) using the Swiss DolorClast®.
When performed properly, RSWT with the Swiss DolorClast® has only minimal risks. Typical device-related non-serious adverse events are:
- Pain and discomfort during and after treatment (anaesthesia is not necessary)
- Reddening of the skin
- Petechia
- Swelling and numbness of the skin over the treatment area
These device-related non-serious adverse events usually disappear within 36h after the treatment.
Treatment Procedure
Clinical Proof
Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial.
Rompe JD, Nafe B, Furia JP, Maffulli N
Am J Sports Med 2007;35:374-383
A series of controlled trials analyze the functioning of different methods in the handling of chronic tendinopathy of the main body of tendo Achillis.
Eccentric loading, versus eccentric loading plus shock-wave treatment for midportion Achilles tendinopathy: A randomized controlled trial.
Rompe JD, Furia JP, Maffulli N
J Bone Joint Surg Am 2009;37:463-470
If the effectiveness of eccentric loading and extracorporeal shock wave therapy separately has been proven, this study aims at knowing if the combination of both these methods can improve results in patients suffering from midbody Achilles tendinopathy.
View Study →
Recommended Settings
| Recommended Settings | Treatment | Myofascial Therapy |
| Number of treatment sessions | 3 to 5 | 3 to 5 |
| Interval between two sessions | 1 week | 1 week |
| Air pressure Evo Blue® | 2 to 4 bar | 3 to 4 bar |
| Air pressure Power+ | 1.5 to 3 bar | 2 to 4 bar |
| Impulses | 2000 on the painful spot |
2000
|
| Frequency | 8Hz to 12Hz | 12Hz to 20Hz |
| Applicator | 15mm | 36mm |
| Skin pressure | Moderate 3 sides of the tendon | Moderate to Heavy |
Contraindications
The following contraindications of RSWT using the Swiss DolorClast® must be considered:
- Treatment over air-filled tissue (lung, gut)
- Treatment of pre-ruptured tendons
- Treatment of pregnant women
- Treatment of patients under the age of 18 years (except for Osgood-Schlatter disease and muscular dysfunction in children with spastic movement disorders)
- Treatment of patients with blood-clotting disorders (including local thrombosis)
- Treatment of patients treated with oral anticoagulants
- Treatment of tissue with local tumors or local bacterial and/or viral infections
- Treatment of patients treated with cortisone
Some indications may not be approved in the United States of America, under regulation by the US FDA. Please refer to the respective Instructions for Use.
