
Lower back pain
Pathology
The social and economic impact of ILBP is substantial. It is the most frequent cause of disability for people under age 45. Acute ILBP (lasting three to six weeks) usually resolves in several weeks, although recurrences are common and low-grade symptoms are often present years after an initial episode.
Its reported point prevalence is as high as 33%, its one-year prevalence as high as 73% and its lifetime prevalence exceeds 70% in most industrialized countries, with an annual incidence of 15-20% in the United States.
In physically active adults not seeking medical attention, the annual incidence of clinically significant ILBP with functional impairment is approximately 10-15%. An alarming increase in the prevalence of chronic ILBP has been observed in industrialized countries over the last years, affecting both men and women and across all ages and racial and ethnic groups.
Risk factors for the development of disabling chronic or persistent ILBP (variously defined as lasting more than three months or more than six months) include pre-existing psychological distress, disputed compensation issues, other types of chronic pain and job dissatisfaction. Diagnosis is based on clinical features. Diagnostic imaging should be considered to rule out other causes of lower back pain (particularly in chronic cases) or to establish the diagnosis of ILBP when in doubt.
The goals of management for patients with ILBP are to:
- Decrease the pain
- Restore mobility
- Hasten recovery so the patient can resume normal daily activities as soon as possible
- Prevent development of a chronic recurrent condition
- Restore and preserve physical and financial independence and comfort
However, management for patients with ILBP is challenged by the following problems:
- Most back pain has no recognizable cause
- An underlying systemic disease is rare
- Most episodes of back pain are unpreventable, and, most importantly
- Few if any treatments have been proven effective for ILBP
Among those treatments are limited bed rest, exercise, nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen (Tylenol), muscle relaxants and opioids (if acetaminophen or NSAIDs do not relieve the pain), chirotherapy, physiotherapy and, ultimately, surgery (in cases of cauda equina syndrome, infections, tumours and fractures compressing the spinal cord, mechanical instability of the back, and, perhaps, intractable pain with a positive straight-leg-raising test and no response to conservative therapy).
However, the analgesic effects of many treatments for non-specific low back pain are small and do not differ in populations with acute or chronic symptoms.
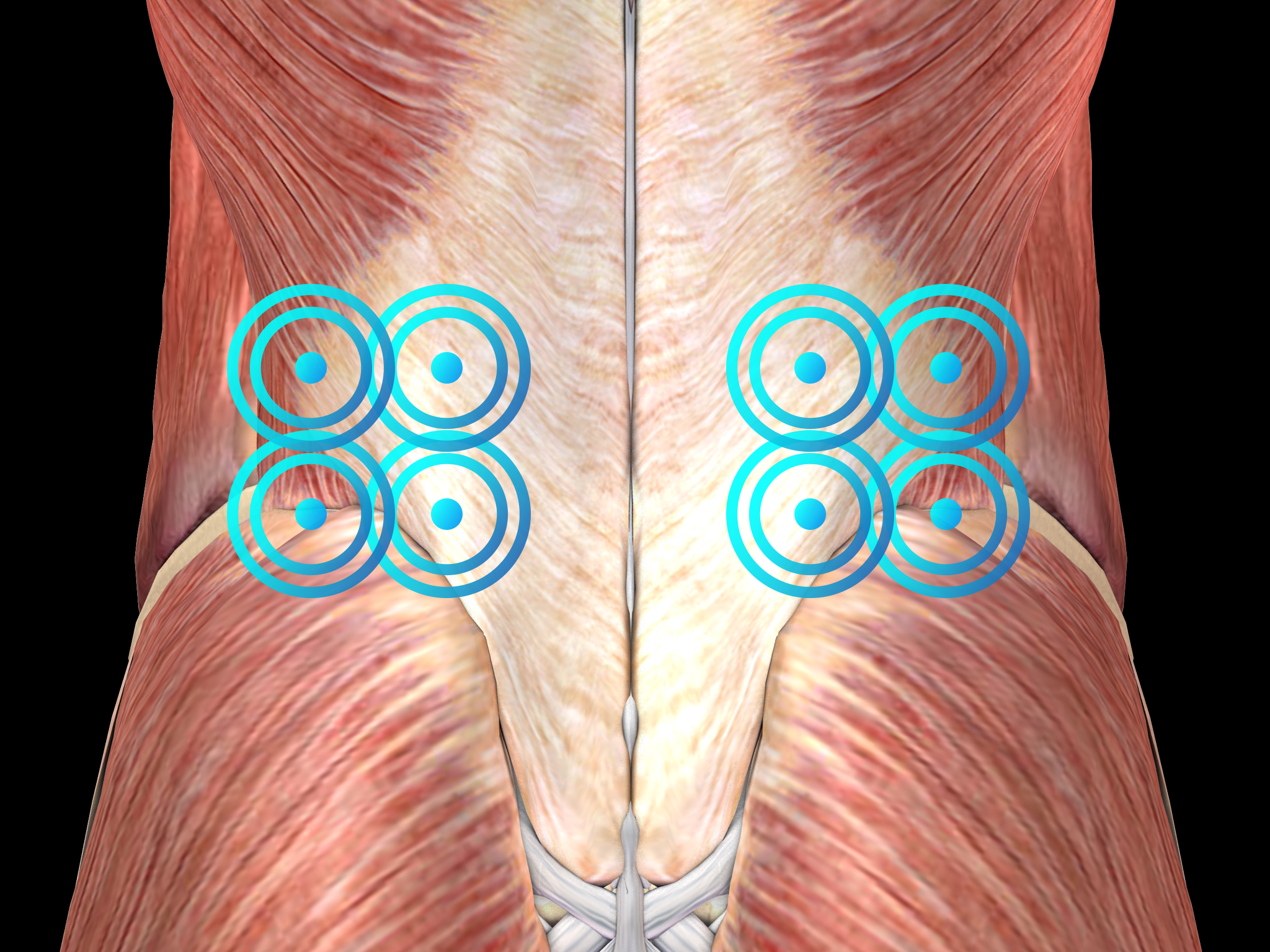
Radial shock wave therapy is an alternative for lower back pain treatment and should be applied before considering surgery.
Side effects of Radial Shock Wave Therapy (RSWT) using the Swiss DolorClast®.
When performed properly, RSWT with the Swiss DolorClast® has only minimal risks. Typical device-related non-serious adverse events are:
- Pain and discomfort during and after treatment (anaesthesia is not necessary)
- Reddening of the skin
- Petechia
- Swelling and numbness of the skin over the treatment area
These device-related non-serious adverse events usually disappear within 36h after the treatment.
Treatment Procedure
Recommended Settings
| Recommended Settings | Treatment | Myofascial Therapy |
| Number of treatment sessions | 3 to 5 | 3 to 5 |
| Interval between two sessions | 1 week | 1 week |
| Air pressure Evo Blue® | 1.5 to 3 bar | 3 to 4 bar |
| Air pressure Power+ | Not recommended | Not recommended |
| Impulses | 2000 on the painful spot |
2000
|
| Frequency | 8Hz to 12Hz | 12Hz to 20Hz |
| Applicator | 15mm | 36mm |
| Skin pressure | Light | Light to moderate |
Contraindications
The following contraindications of RSWT using the Swiss DolorClast® must be considered:
- Treatment over air-filled tissue (lung, gut)
- Treatment of pre-ruptured tendons
- Treatment of pregnant women
- Treatment of patients under the age of 18 years (except for Osgood-Schlatter disease and muscular dysfunction in children with spastic movement disorders)
- Treatment of patients with blood-clotting disorders (including local thrombosis)
- Treatment of patients treated with oral anticoagulants
- Treatment of tissue with local tumors or local bacterial and/or viral infections
- Treatment of patients treated with cortisone
Some indications may not be approved in the United States of America, under regulation by the US FDA. Please refer to the respective Instructions for Use.
