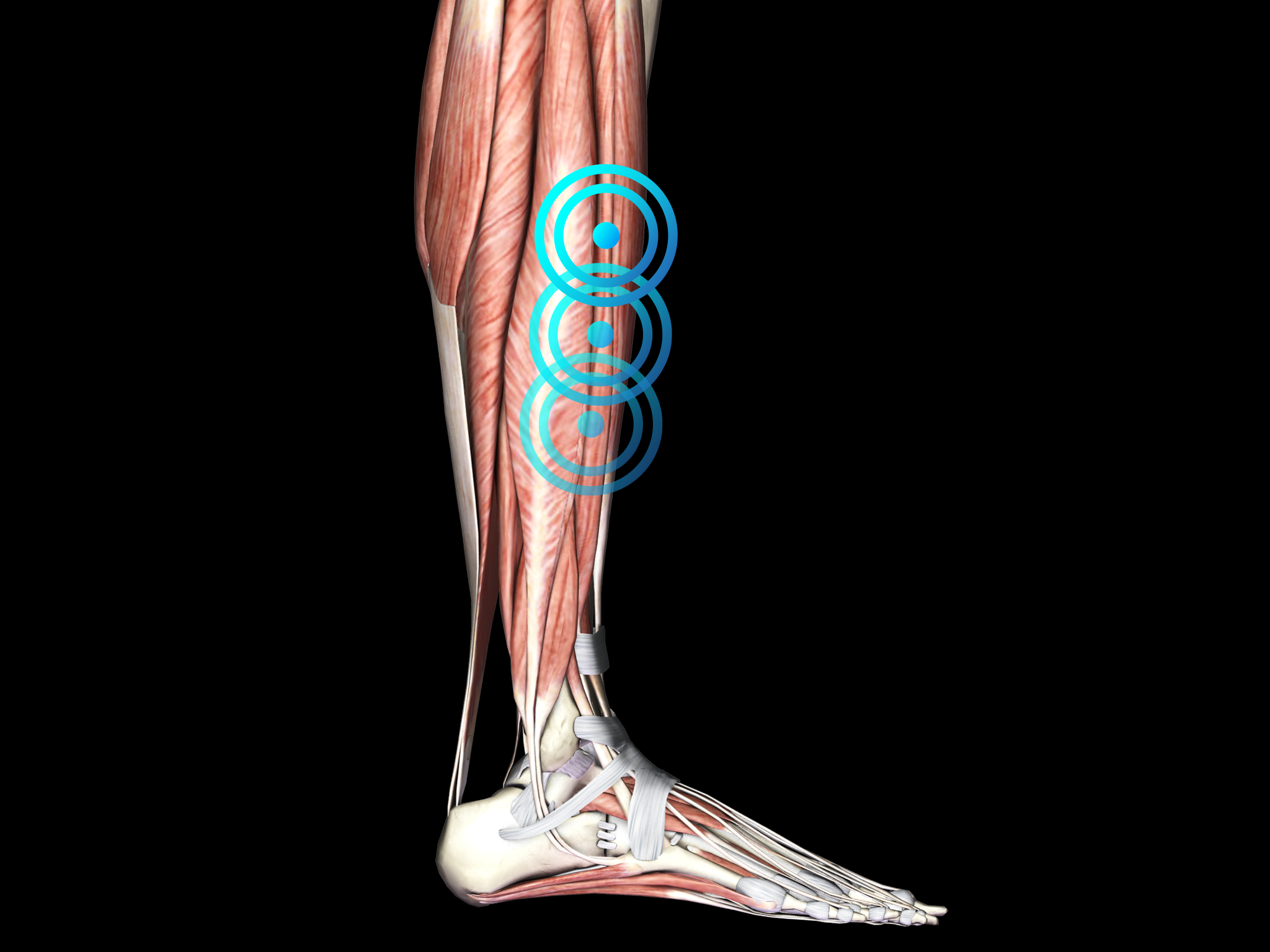
MEDIAL TIBIAL STRESS SYNDROME
Medial tibial stress syndrome (MTSS) - commonly known as ‘‘shin splints” – is a frequent overuse injury or repetitive-stress injury of the lower extremity.
Pathology
The condition is one of the most common causes of exertional leg pain in athletes, and usually presents as diffuse pain of the lower extremity, along the middle-distal tibia associated with exertion. Early courses of MTSS are characterized by pain that
- Gets worse at the beginning of exercise,
- Gradually subsides during training, and
- Stops within minutes after exercise.
Later, pain may present with less activity and may even occur at rest.
Diagnosis is based on the clinical features of the disease. Diagnostic imaging should be considered to rule out other causes of exertional leg pain or to establish the diagnosis of MTSS when in doubt.
Training errors (“too much, too fast”) appear to be the most common factors involved in MTSS.
The condition is most often found in runners, soccer and basketball players, and in dancers. Notably, MTSS is almost always associated with biomechanical abnormalities of the lower extremity including knee abnormalities, tibial torsion, femoral anteversion, foot arch abnormalities, or a leg-length discrepancy.
However, improper footwear (including worn-out shoes) can also contribute to shin splints. A variety of tibial stress injuries can be involved in MTSS including tendinopathy, periostitis, and dysfunction of the tibialis posterior, tibialis anterior and soleus muscles. Women appear to be more affected than men and have an approximately threefold risk for progression to stress fractures.
The treatment of MTTS should start with rest and ice in the acute phase, followed by low-impact and cross-training exercises during rehabilitation and a modified training program (decreased intensity, frequency, and duration, regular stretching and strengthening exercises, wearing proper-fitting shoes with good shock absorption). Orthotics, manual therapy, injections, and acupuncture may also help to alleviate the symptoms.
Patients not responding to conservative treatment for six months should then undergo radial shock wave therapy for medial tibial stress syndrome treatment.
Surgery should also be considered for recalcitrant cases of MTSS.
Side effects of Radial Shock Wave Therapy (RSWT®) using the Swiss DolorClast®
When performed properly, RSWT® with the Swiss DolorClast® has only minimal risks. Typical device-related non-serious adverse events are:
- Pain and discomfort during and after treatment (anesthesia is not necessary)
- Reddening of the skin
- Petechia
- Swelling and numbness of the skin over the treatment area
These device-related non-serious adverse events usually disappear within 36h after the treatment.
Treatment Procedure
Recommended Settings
| Recommended Settings | Treatment | Myofascial Therapy |
| Number of treatment sessions | 3 to 5 | 3 to 5 |
| Interval between two sessions | 1 week | 1 week |
| Air pressure Evo Blue® | 2 to 4 bar | 3 to 4 bar |
| Air pressure Power+ | 1.5 to 3 bar | 2 to 4 bar |
| Impulses | 2000 on the painful spot |
2000
|
| Frequency | 8Hz to 12Hz | 12Hz to 20Hz |
| Applicator | 15mm | 36mm |
| Skin pressure | Moderate 3 sides of the tendon | Moderate to Heavy |
Contraindications
The following contraindications of RSWT using the Swiss DolorClast® must be considered:
- Treatment over air-filled tissue (lung, gut)
- Treatment of pre-ruptured tendons
- Treatment of pregnant women
- Treatment of patients under the age of 18 years (except for Osgood-Schlatter disease and muscular dysfunction in children with spastic movement disorders)
- Treatment of patients with blood-clotting disorders (including local thrombosis)
- Treatment of patients treated with oral anticoagulants
- Treatment of tissue with local tumors or local bacterial and/or viral infections
- Treatment of patients treated with cortisone
Some indications may not be approved in the United States of America, under regulation by the US FDA. Please refer to the respective Instructions for Use.
