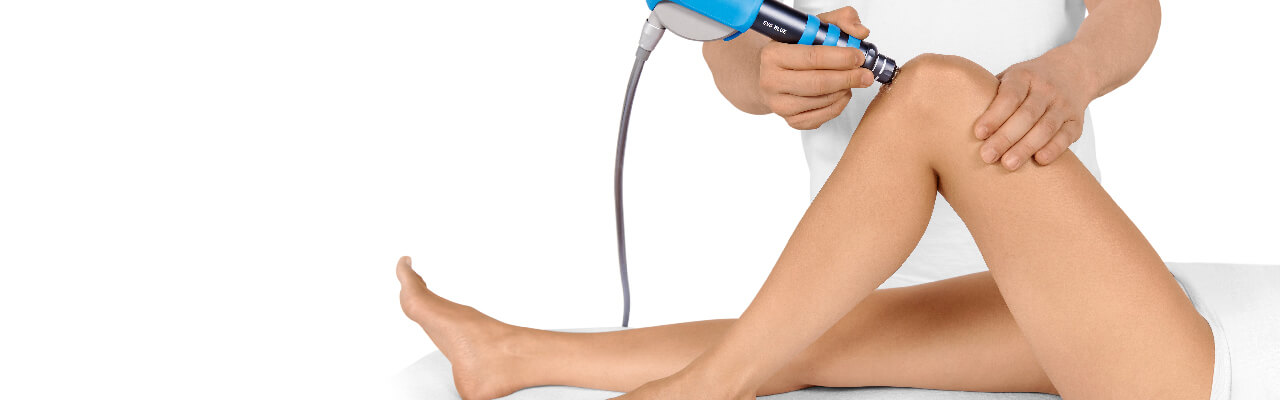
OSGOOD-SCHLATTER DISEASE
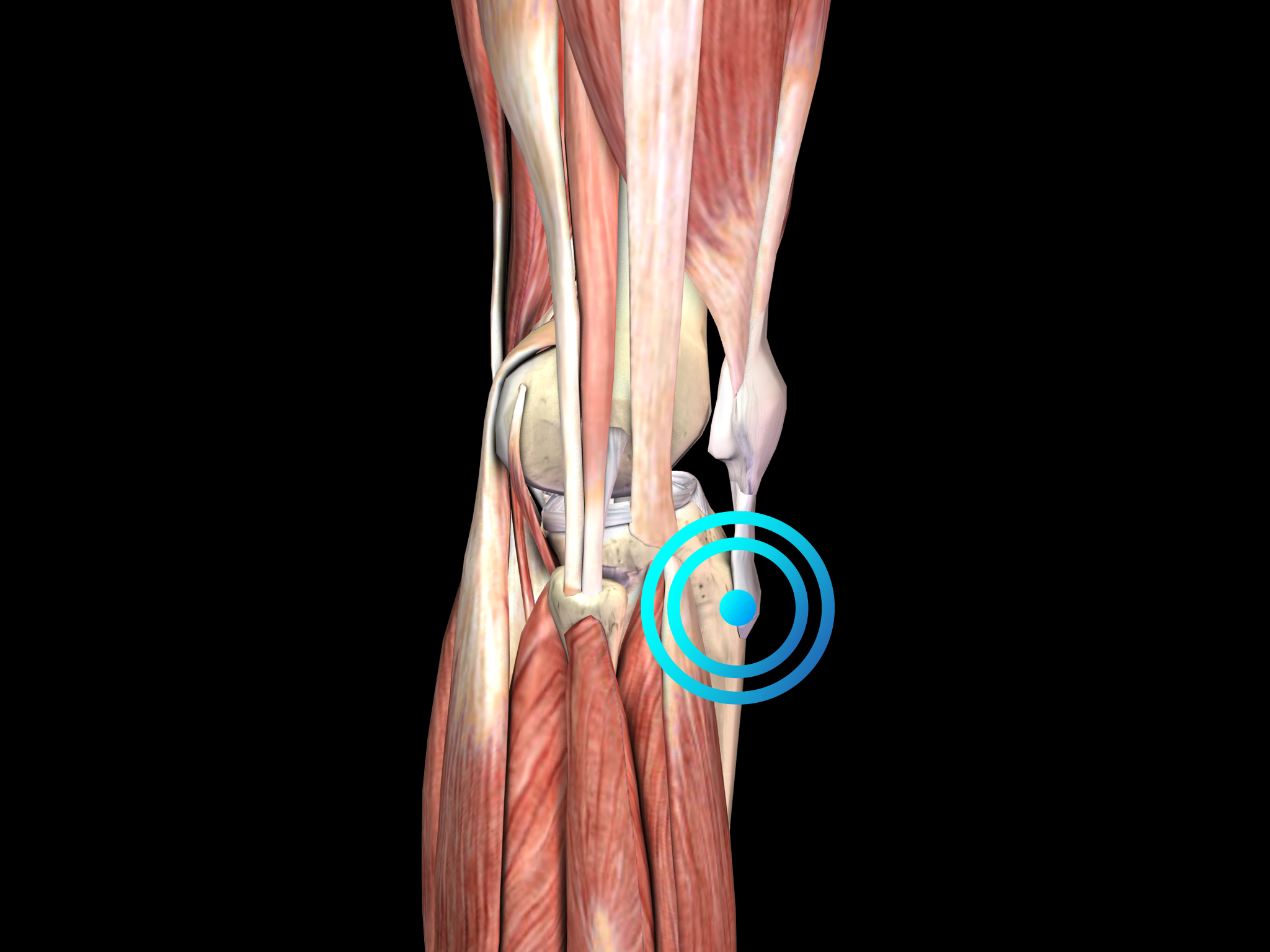
Pathology
Osgood-Schlatter disease is thought to be caused by repetitive strain and chronic avulsion of the secondary ossification center of the tibial tuberosity, i.e., by small injuries due to repeated overuse before the area has finished growing. The repetitive strain is from the strong pull of the quadriceps muscle produced during sporting activities, particularly during running, jumping and climbing. Accordingly, Osgood-Schlatter disease is common in adolescents who play soccer, basketball and volleyball, and who participate in gymnastics. The tibial tuberosity avulsion continues to grow, ossify and enlarge. The intervening area may become fibrous, creating a localized nonunion, or may show complete bony union with mild enlargement of the tibial tuberosity. In any case, the result is a traction apophysitis of the tibial tubercle.
Diagnosis is based on the clinical features of the disease and on diagnostic imaging. Particularly in unilateral cases of Osgood-Schlatter disease, plain radiographs of the knee are recommended to rule out other conditions such as acute tibial apophyseal fracture, infection, or tumor.
The true incidence of Osgood-Schlatter disease is unknown. The predominant age is between 12 and 15 years in boys and between 8 and 12 years in girls, coinciding with periods of growth spurts. Boys are more affected than girls (approximately 3:1). In 20-30% of all cases OSM presents bilaterally.
The treatment of Osgood-Schlatter disease should start with conservative treatment modalities including rest, icing, modification of activities, and rehabilitation exercises.
Patients not responding to conservative treatment for six months (approximately 10% of all patients) should then undergo radial shock wave therapy for Osgood-Schlatter disease treatment.
Surgery should be considered for recalcitrant cases of Osgood-Schlatter disease in skeletally mature patients, aiming at surgical excision of the ossicle (in case of a localized non-union) and/or free cartilaginous material.
Side effects of Radial Shock Wave Therapy (RSWT) using the Swiss DolorClast®.
When performed properly, RSWT with the Swiss DolorClast® has only minimal risks. Typical device-related non-serious adverse events are:
- Pain and discomfort during and after treatment (anaesthesia is not necessary)
- Reddening of the skin
- Petechia
- Swelling and numbness of the skin over the treatment area
These device-related non-serious adverse events usually disappear within 36h after the treatment.
Treatment Procedure

Palpate
Locate the area of pain through palpation and biofeedback.

Mark
Mark the area of pain.

Apply gel
Apply coupling gel to transmit shock waves to the tissue.

Apply shock waves
Deliver Radial or Focused Shock Waves to the area of pain while keeping the applicator firmly in place on the skin.
Clinical Proof
Extracorporeal shockwave therapy in the treatment of the osteochondropathy of tibial bone roughness.
Titov VV, Litvinenko A
Abstracts 10th International Congress of the International Society for Musculoskeletal Shockwave Therapy, Toronto, Canada, 2007, pp. 46-47
Recommended Settings
| Recommended Settings | Treatment |
| Number of treatment sessions | 3 to 5 |
| Interval between two sessions | 1 week |
| Air pressure Evo Blue® | 2 to 4 bar |
| Air pressure Power+ | 1.5 to 3 |
| Impulses | 2000 on the painful spot |
| Frequency | 8Hz to 12Hz |
| Applicator | 15mm |
| Skin pressure | Light to Moderate |
Contraindications
The following contraindications of RSWT using the Swiss DolorClast® must be considered:
- Treatment over air-filled tissue (lung, gut)
- Treatment of pre-ruptured tendons
- Treatment of pregnant women
- Treatment of patients under the age of 18 years (except for Osgood-Schlatter disease and muscular dysfunction in children with spastic movement disorders)
- Treatment of patients with blood-clotting disorders (including local thrombosis)
- Treatment of patients treated with oral anticoagulants
- Treatment of tissue with local tumors or local bacterial and/or viral infections
- Treatment of patients treated with cortisone
Some indications may not be approved in the United States of America, under regulation by the US FDA. Please refer to the respective Instructions for Use.
